Plans
Next steps, financing & exit strategy
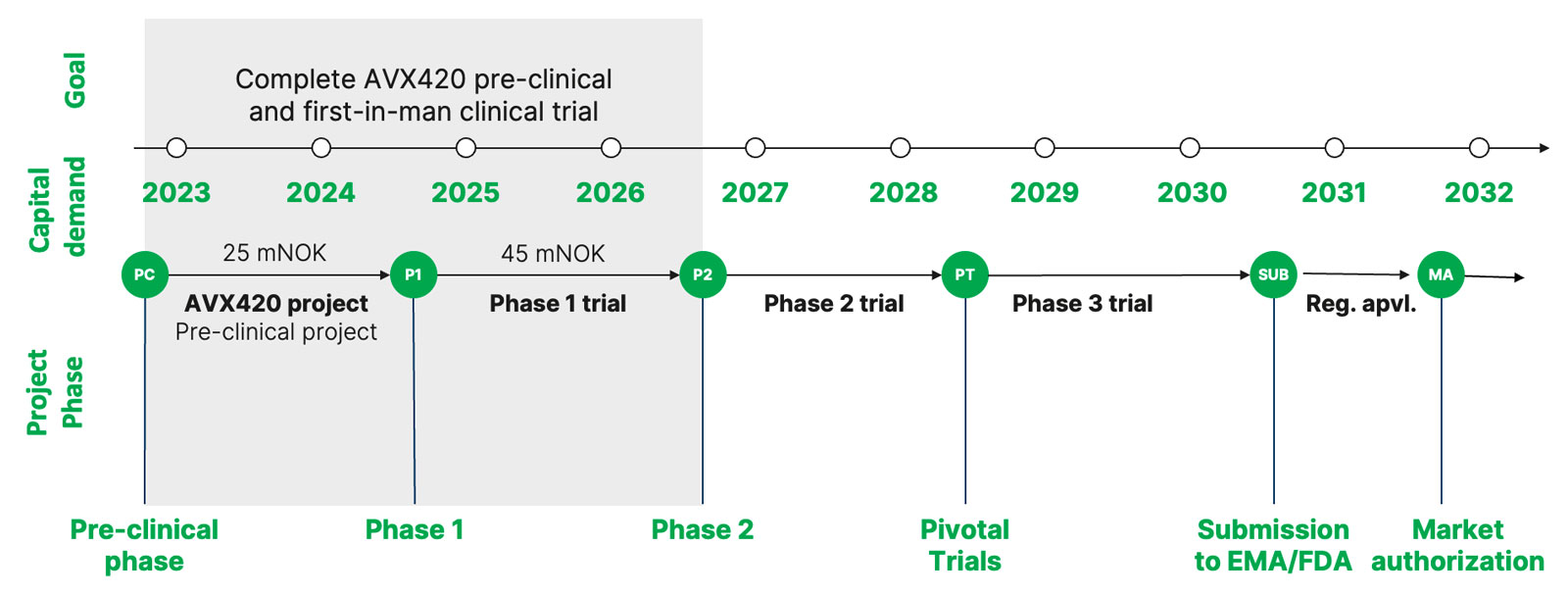
Roadmap until 2026
2022: The Norwegian company Avexxin Oncology AS continues the ongoing research as well as formulation optimization
2023: Finalize intravenous formulation development for clinical use and initiate mandatory preclinical testing to demonstrate that AVX420 is safe for testing in humans
2023: Decide on the first cancer indication to focus the initial clinical development efforts on (e.g. leukemia or breast cancer)
2024: First patient in phase 1 clinical first-in-man testing in cancer patients
2026: Key results of phase 1 first-in-man
Financing
Pre-clinic
– Total capital requirement for completion of the pre-clinic and formulation for administration to humans totals 25 mNOK.
– The company has applied for 16 mNOK in research support from the Norwegian Research Council. A response to the application is expected end of 2022. If the research support is granted the remaining financing need is 9 mNOK.
Phase 1 trial
– The total capital requirement for the implementation and completion of the phase 1 study amounts to 45 mNOK. – The goal is to identify a VC or co-development partner for financing the phase 1 study.
Exit strategy
If the phase 1 trial reaches positive safety outcomes and signs of efficacy, Avexxin Oncology AS believe that a significant value of the asset will be in scope and we may aim for an early and attractive exit.